
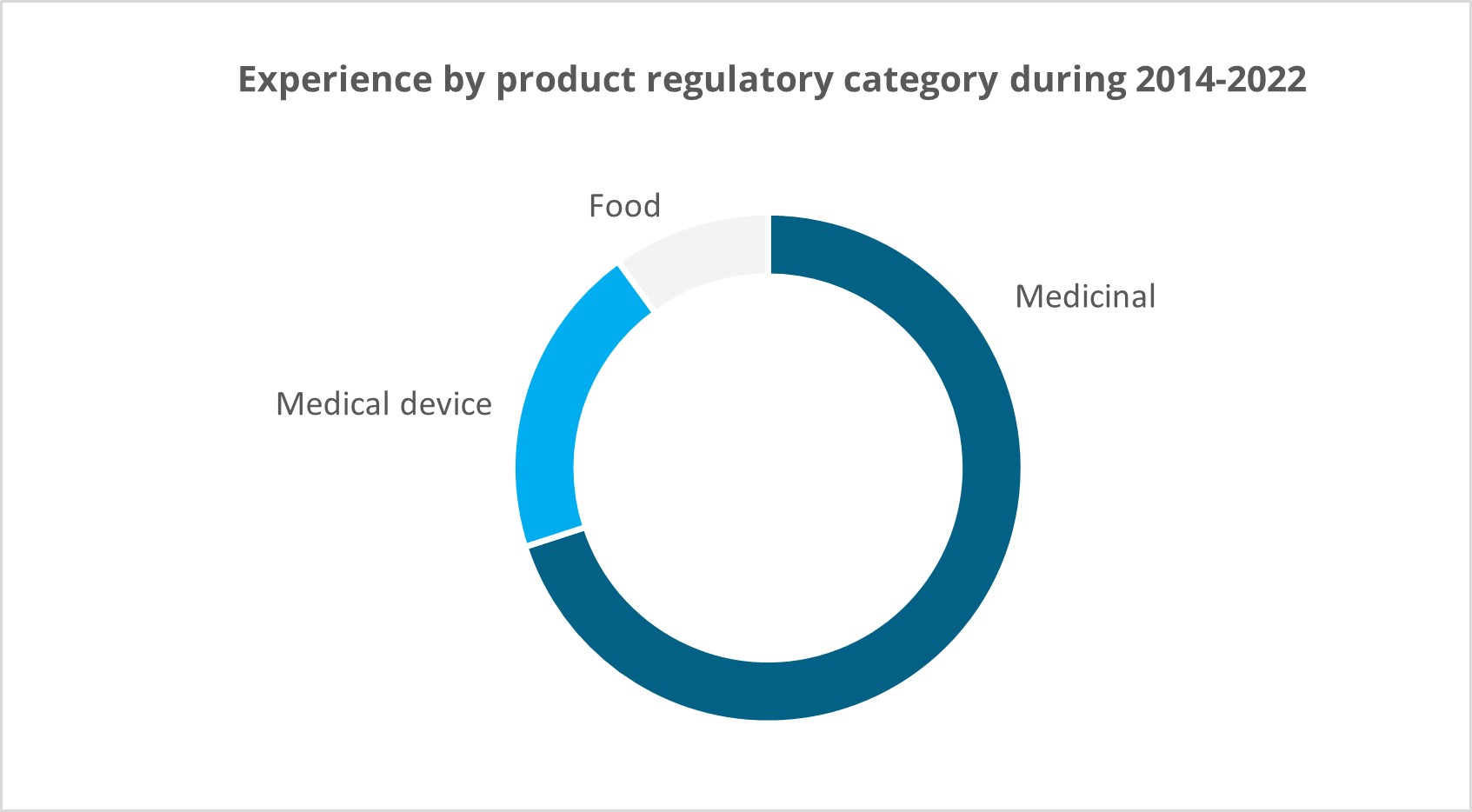
Clinical trial services for medical devices, food products and medicinal products
Medfiles offers comprehensive clinical services for medical devices, in vitro diagnostic devices, food products and medicinal products. We support sponsors from early phase pilot studies to post-market studies. We provide customised solutions as well as complete packages from study planning to final reporting. Our one-stop solution includes project coordination, medical writing, submissions and registrations, study set-up activities, essential study documents, monitoring, data management and statistics and final study reports.
Throughout the clinical trial process, our team of highly experienced clinical project managers (CPMs) and clinical research associates (CRAs) ensures that your projects are completed on schedule and within the budget while complying with international quality standards. In addition to the Nordic and Baltic countries, we support clinical trials also in other European countries through our network of experts. Contact us
Strong clinical trial experience and competence
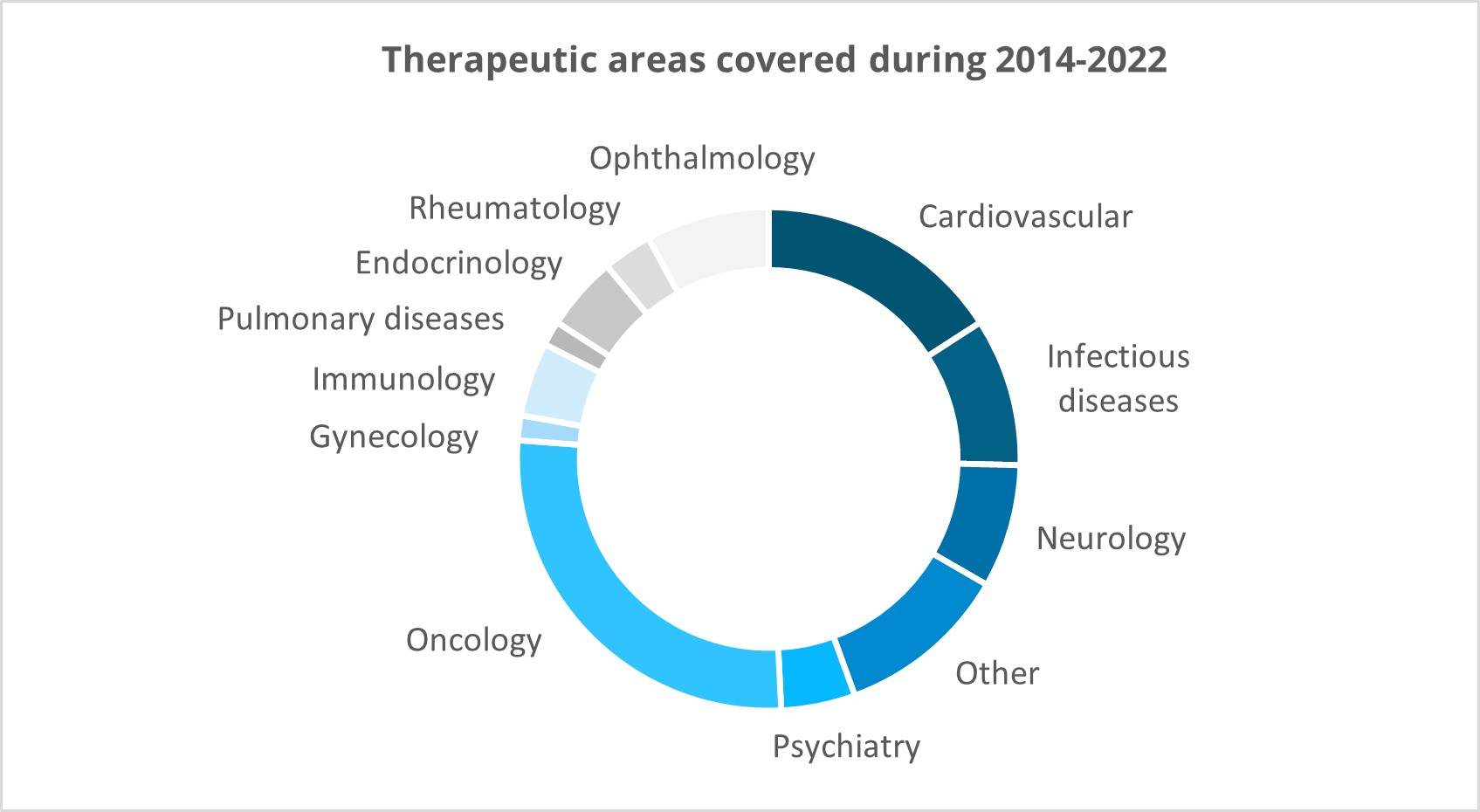
We provide continuous training for our employees and carefully follow the development of regulations to ensure the highest quality and compliance of our services. Our core competence is in clinical investigations on medical devices and dietary interventions.
30+
Year’s experience
1000+
TRIAL SITES
320+
CLINICAL TRIALS
150 000+
SUBJECTS
Extensive experience from various therapeutic areas:
Clinical trial services

Project management in clinical trials
Medfiles will carefully assign clinical project managers (CPM) with proven expertise in clinical research to your project. The CPM assigned to your project will provide ongoing consultation and coordination and communicate all aspects of the trial as defined by you. The CPM is the key contact for all parties involved in the clinical trial and acts as the key contact between the sponsor and Medfiles.
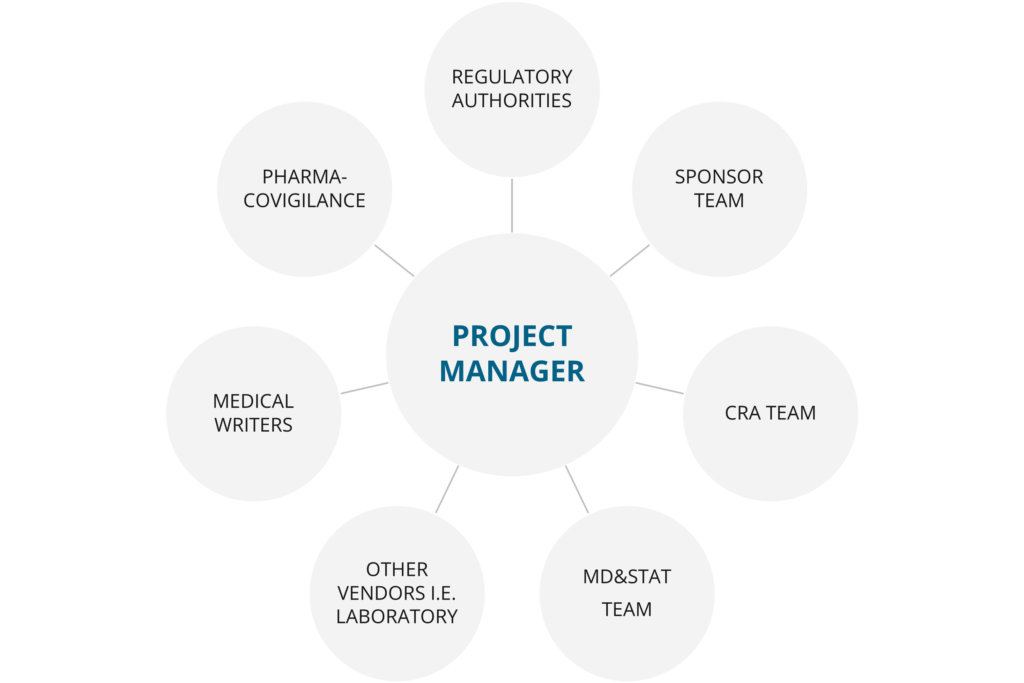
Our dedicated CPMs perform a variety of functions, such as:
- ensuring the progress of the trial
- serving as the primary resource and point of communication for the sponsor and project team
- effectively utilising our global partners and technical resources, includig eCRF, eTMF and EMR providers
- optimising your site selection and feasibility analysis
- leading and managing the project team, including identifying and tracking for time schedules, quality and cost of project
- identifying, documenting and mitigating risks early in the process to address potential obstacles
- applying a proactive approach to develop a project team focused on quality and efficiency
- communicating issues and solutions
- projecting “what if” scenarios
- keeping in mind and managing the “big picture” of the trial, the protocol and the sponsor’s objectives
- coordinating and managing the IEC and regulatory submissions.

Clinical trial set-up and initiation
You will receive focused, one-on-one interaction and streamlined, responsive communication with experts who can help you with all aspects of trial set-up and initiation. Our medical writers can step in already at the study planning stage. Engagement and qualification of professional sites and investigators are crucial elements in clinical trials. With our large network of trial infrastructure in the Nordic and Baltic areas, we can ensure high-quality performance of sites.
Clinical trial planning
- Assistance with study design
- Medical writing for clinical trial protocols and investigation plans
- Protocol review and plans from regulatory and GCP perspectives
- Trial scheduling
- Consultation on regulatory requirements (local, EMA, EFSA) and GCP (EMA, EFSA) requirements
Site identification and qualification
- Clinical trial protocol and plan feasibility
- Principal investigator (PI) and site identification
- Site qualification visits
- Site selection with sponsor
- Feasibility and qualification documentation
Study initiation
- Project kick-off and investigator meetings
- Trial and organisation registrations, such as EU portals CTIS and EFSA and ClinicalTrial.gov
- Translations and local adaptations, such as informed consents, study synopsis and patient diaries
- Submissions
- IEC and regulatory submissions for non-pharma and non-interventional studies
- Part I and Part II CTIS submissions
- Study management documents, such as project plans, Trial Master File plans and (eTMF) set-up, monitoring plans and investigator site file (ISF) preparation
- Set up of vigilance activities
- Data management (eCRF planning, set-up and review) and statistical plans
- Site and other vendor contracts and negotiations
- Site initiation visits
- Site staff training and support

Clinical trial monitoring
Medfiles matches the right team to the clinical program of your medicinal product, medical device or food product to ensure the highest quality data review and site support. We have a strong Nordic and Baltic presence in Finland, Sweden, Estonia, Latvia and Lithuania and regionally-based clinical research associates (CRAs) in our European network. Our clinical project managers and CRAs are experienced and comprehensively trained, with in-depth knowledge of clinical trial monitoring procedures, regulatory guidelines and a wide range of therapeutic areas.
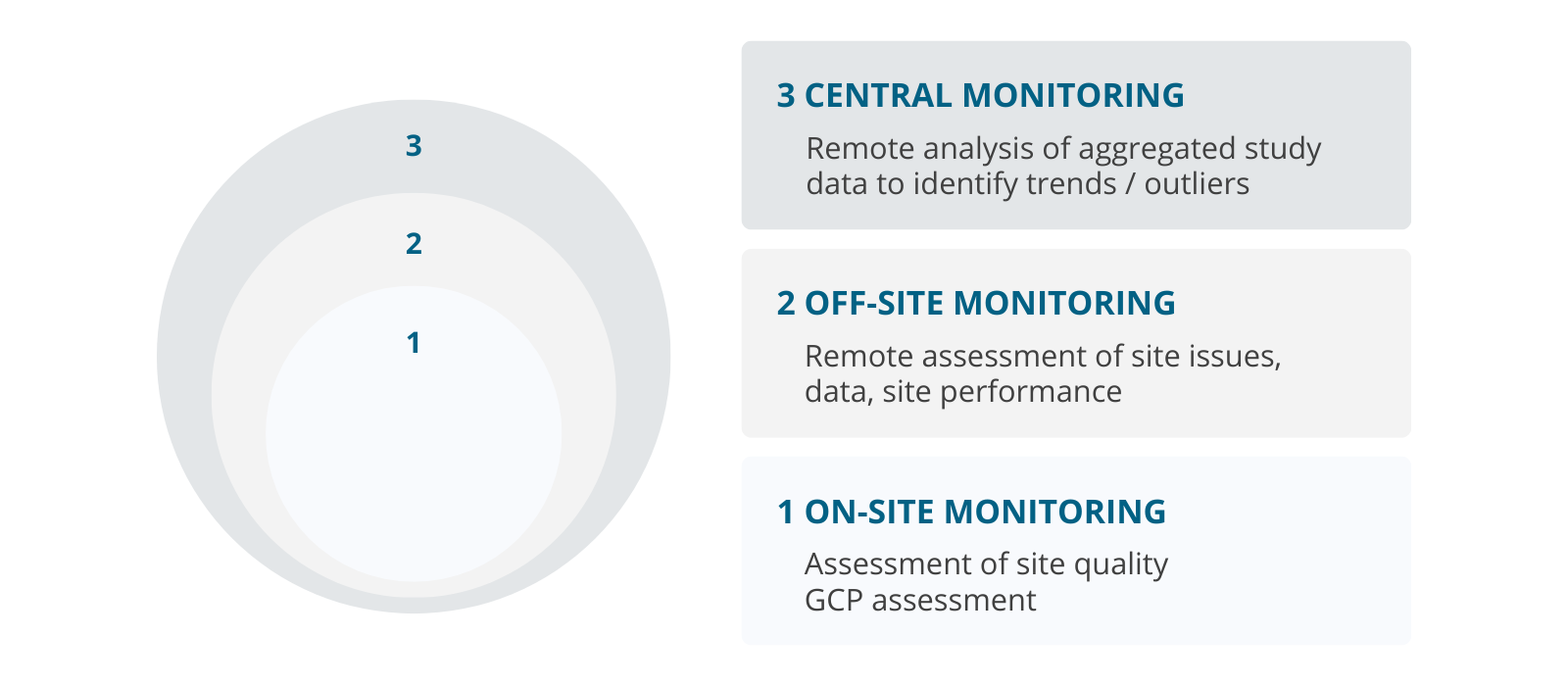
Assurance of GCP compliance
Monitoring strategy
- on-site and remote monitoring
- centralised monitoring
- uniform practices
- remote evaluation of accumulated data
- risk-based monitoring
- risk evaluation-based monitoring
- dynamic process
- signal detection
- scientific consideration
Trial documents
- collection of essential documents
- TMF maintenance
- Agatha eTMF
- ISF maintenance
- updates of trial-related plans and instructions
IEC and regulatory updates
- submission of substantial modifications and amendments
- trial status updates e.g. in CTIS portal
- liasion with IEC and other competent authorities
Vigilance
- AE and SAE collection and processing
- development safety update reports (DSUR)
- data and safety monitoring board (DSMB)
- medical coding
- validated safety database
- reconciliation of trial and safety databases
Read more
Data management
- data handling (electronic CRFs)
- query handling

End of clinical trial activities
The most exciting phase of the trial comes once the database is closed and the project is approaching the reporting phase. It is important that both the conduct of the study as well as the obtained results are documented as required from the scientific and regulatory point of view.
- Site close-out visits
- End of study notifications for IEC and regulatory authorities
- TMF delivery
- Vigilance activities
- Data cleaning, data base lock procedures and statistical analysis via vendor
- Clinical trial reports
- interim reports
- final reports
- tables and listings
- Scientific publications
- Archiving arrangements
Medfiles is a research partner that invests in your results and is dedicated to the success of your research. By selecting us as your research partner, you can be sure that the research will be tailor-made, but conducted in accordance with legislation and good clinical practice. Ask for help with individual project stages or the full study solution!

Free Guide: Quick tips for successful CTIS submissions
The CTIS portal brings new responsibilities and requirements and raises questions. We created a practical guide on how to successfully submit your clinical trial applications. The information is especially targeted at academic researchers conducting clinical trials in Finland.
Free Guide: HOW TO MEET MDR GOOD CLINICAL PRACTICE REQUIREMENTS IN PRACTICE?
Do you work with medical devices and need information on the MDR/ISO 14155:2020 good clinical practice requirements? The MDR states that clinical investigations must be performed according to good clinical practice. In addition to observing the requirements set in the MDR itself, sponsors must also be aware of the requirements set in the ISO standard. We have created a guide on what you need to know about these requirements regarding the clinical trial set-up, monitoring and ending phase.
clinical team leaders

Essi Sarkkinen
Director, Development & Operations
Essi Sarkkinen has worked at Medfiles since 2014, first leading the Food & Feed and Cosmetics Unit and for the past five years, leading the Medfiles Clinical Unit having experts in Finland and the Baltics. In the beginning of 2024, she was nominated as a Director of Development & Operations.
Essi has a PhD in clinical nutrition and she is adjunct professor at the University of Eastern Finland. Essi has published dozens of scientific articles in peer-reviewed journals and supervised four academic dissertations. After her academic career, she has worked for over 15 years managing and leading contract research in the field of food and nutrition. In the beginning of the 2000s, Essi started working with clinical trials for medicinal products and good clinical practice (GCP), as well as regulatory affairs concerning food products, including work with health claims and novel foods.
Essi has over 30 years of experience in clinical trials and medical writing as well as know-how of R&D and regulatory affairs in the fields of food, medical devices and pharmaceuticals. All in all, she has worked in various leadership positions in contract research organisations for over 25 years.

Satu Koskimies
Head of Clinical Operations
Satu Koskimies has worked at Medfiles since 2022, first as a clinical project manager and from the beginning of 2023, as Head of Clinical Operations. Satu has a M.Sc. in biology, and she is also a practical paediatric nurse and a seasoned clinical trial professional.
Since 2001, Satu has accumulated more than two decades of experience in clinical project management and coordination, medical writing and regulatory intelligence.
At Medfiles, she is involved with both medicinal products and medical devices, with a history of previously working with various CROs and biotech and pharma companies. Actively participating in the creation and implementation of robust quality management systems, preparing new operating procedures and enhancing high-quality performance in clinical research activities have been her favourite assignments during her career.
As Head of Clinical Operations, Satu supports and leads the Clinical Team at Medfiles, consisting of skilled, competent and dedicated clinical project managers and clinical research associates.
Contact us
You may also be interested in:
- Clinical investigation and performance evaluation services for medical devices
- Clinical study services for food products
- Clinical trial services for medicinal products
- Medical writing
- Guide: How to meet MDR Good Clinical Practice requirements in practice?
- Meet our people: CRA ensuring success of clinical trials
- Free guide: Quick tips for successful CTIS submissions
- 10 tips for a successful investigator-initiated study (IIS)
